
Direct Transfer from A High Shear Granulator To a Fluidized Bed Dryer
Source : Yenchen Technical Process Consultant, Dr. Fred Rowley
When processing a dangerous or potent drug, direct transfer from a high shear granulator to a fluidized bed dryer is mandatory. Doing this minimizes operator exposure and reduces the quantity of powder lost to the room during manual transfer. So, if you purchase a direct transfer system for this purpose the justification is straightforward.
But what if you are processing multiple products with minimum exposure risk and with a low profit margin? Many companies find it difficult to easily justify this expense.
There is a defendable justification. It takes a little time to explain.
It begins with discussing drug substances in general and the Pharmaceutical industry. It goes back in history to the development of the rotary tablet press. To increase productivity, etc. etc. Originally, granulating drug powder was performed so that the powder flow could keep up with the tablet press; not to obtain better content uniformity. A classical problem that we still see today as see in Photo 1.

Photo 1. Manual de-lumping of Metoprolol wet massing before oven drying
The granulated material was wet and needed to be dried so that it would flow and be compressed into a tablet. We used an oven. And you can do many things while you load the trays: remove the lumps, screen the materials, uniformly distribute the material. There was more art than science as seen below in Figure 1.
Figure 1. Visual illustration of Aspirin Manufacturing, 1957.
When the fluidized bed dryer was introduced to the Pharmaceutical industry in the 1970’s the industry slowly transferred the drying process from the oven to the fluidized bed dryer. Today it is the preferred method of drying. Drugs are more powerful or dangerous in ever increasing small quantities requiring smaller doses in small tablets at ever increasing higher compressing speeds.
There remains one basic problem: how to remove the lumps, screen the materials and uniformly distribute the wet materials in a large bowl? The larger the bowl the bigger the issue. Many firms place a large, gross screen over the bowl as the operator loads the wet material. Firms mill the wet mass as a separate unit operation using a mill without a screen. Some do nothing and allow the dryer to start up with the powder semi-massed against the bottom screen.
Unfortunately, the technical problem begins when the wet mass is allowed to settle before drying and the longer the settling time the larger the potential problem. The fault with conventional thinking is that the post drying milling operation will reduce or eliminate this problem. The literature does not support this position. Further, the problem may take different forms with different drugs and potencies: greater weight variation, erratic sticking/picking, wider content uniformity; especially with smaller tablets. And over time, quantifiable lower batch yields. This also provides a partial explanation for different batches that have the same drug with the same wet massing and having been dried in the same dryer and then compressed using the same tablet press sometimes produce tablets with different results. The opportunity to standardize the drying process should have started as the material entered the product bowl and once lost this opportunity does not return.
The use of a direct product transfer utilizing an in-line mill eliminates large lumps, reduces particle variation and the resulting tendency to aggregate after transfer and before the drying process begins. Note photo 2 below. Casual observation confirms this as many powders tend to begin the drying process before the product transfer is completed and the formal drying process begins. This seemingly small additional benefit to operator safety is in fact an assignable product variability root cause.

Photo 2. Mass transfer of granulation through a mill
Analyzing the situation further the scientist sees that different drug substances might require individual responses to this potential problem. Some products do not lump, some form large rocks; some harden with time and some perform well without any pre-drying processing. The conclusion must then be partially dependent upon your product mix, the value of the drug, the danger of the drug and how well your product performs on the tablet press. Even with non-potent drugs and larger the tablet; the larger the product mix the more likely you will see lower batch yields when a direct transfer based granulating process is compared head to head with a more manual procedure.
And this is quantifiable with an analysis of tablet weight uniformity data and compressing yields of individual products.
If the justification is based on product quality or better compressing yields, then tablet weight uniformity of small, potent drug tablets as well as year over year compressing yields will reveal the true, quantifiable savings seen when using a direct material transfer from the SMG to the fluidized bed dryer.
An analysis of the weight control specification graph below illustrates the analysis. This is a fictional graph from a computer-controlled tablet press. The control is a common three tier control system: do nothing within established weight (pressure) limits, adjust the tablet weight, reject the individual tablet, shut down the tablet press. If the direct transfer method using an in-line powder mill reduces particle size and moisture variability which then results in better weight and content control; then fewer tablets will be rejected (even using tighter reject specifications) and given enough product history, produces higher yields year over year.
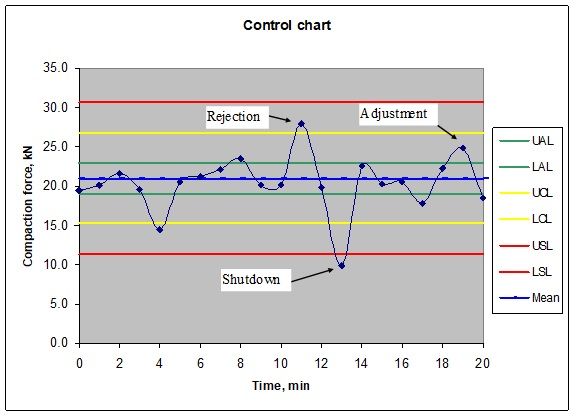
Graph 1. A three level process control graph from a Kikusui Libra tablet press
The conclusion must therefore be that in-line product transfer must be evaluated as a potential process using an analysis of historical batches. In various cases this process will produce better content and weight control during the tablet compressing process.
If you have any technical questions, please contact with Grace Chiang Email:grace.chiang@yenchen.com.tw
Copyright © 2019 YENCHEN MACHINERY CO., LTD. All Rights Reserved.
