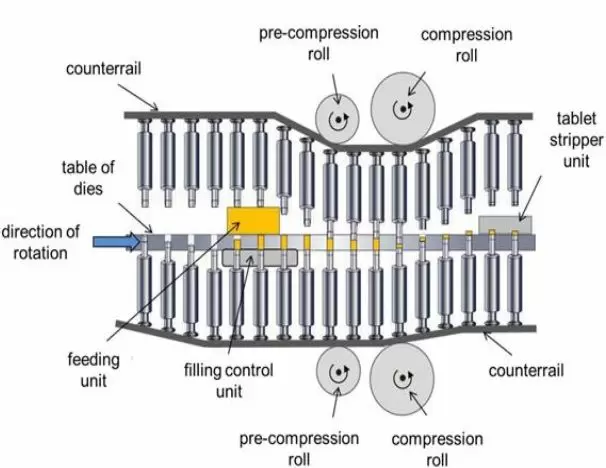
Is Pre-Compression Tablet Thickness A True Critical Operating Parameter? -3
Source : Yenchen Technical Process Consultant, Dr. Fred Rowley
- Use a mathematical ratio/percentage of the final tablet thickness setting.
- Guess based on experience.
- Manually set the pre-compression thickness based on observation with the main/final tablet thickness control disengaged.
Using a straight percentage of the final thickness value or setting (the final "compressing force") is not recommended. Doing this negates the inherent difference between individual batches of the same product.
Guessing based on experience is even worse as this again negates the inherent difference between batches or changes in component vendors or grades of raw materials.
And here we arrive at the central issue. Operating staff frequently duck the issue by not including this control on their master batch records and leaving it up to an operator to adjust the setting.
The accepted method of setting this important control can be described in simple terms:
- Increase ("back off") main/final thickness until the pressure rolls do not move.
- Decrease tablet thickness at the pre-compression station until you barely make a tablet. Tablet thickness can be determined either by physical measurement or visually comparing the tamps to the finished tablets at target thickness stated on the batch record.
In many cases where physical measurement is possible, the tamp should be at least 150% of the final tablet thickness target and may be even thicker in some cases. Further, although not seen in our photograph, most tamps will fall apart with even minimal mechanical agitation. It may not be possible to measure tamp thickness. In this case, a simple photo attached to the batch record will provide adequate documentation. Remember that our goal is to remove excessive air trapped in the powder, not to make a tablet.
As agency inspectors focus on tablet press operation, it is then only a matter of time before the lack of attention to the importance of the pre-compression thickness control becomes an issue. All an inspector has to do to justify an observation is ask: (1) how many batches have an investigation for capping; (2) what are the recognized settings to control/eliminate capping; and (3) what controls do you have on your current master batch record?
If you have any technical questions, please contact with Grace Chiang Email:grace.chiang@yenchen.com.tw
Copyright © 2019 YENCHEN MACHINERY CO., LTD. All Rights Reserved.
