
Introduction of Computer System Validation and 21 CFR Part 11
GAMP, formulated by the ISPE, and GMP are not the same, it does not belong to the mandatory norms and standards, but rather a guide to the theory and practice methodology. Because it does not belong to regulation, and ISPE is not belong to certification authority, the statement in GAMP: Any claims which "has passed GAMP certification" or "has been approved by GAMP" is inappropriate.
Many theories and concepts proposed from GAMP5 are still very scientific and reasonable. Although it is not part of legislation, but it is currently the main reference for computerized system validation in the international pharmaceutical industry, and it is the most important pharmaceutical automation regulatory guide.
Process
Validation of computer systems is not only limited to the use of the process system. Validation of the new system should be begun from the definition and design phase of the system and ended at useless stage. System Validation Life Cycle (SVLC) should be accompanied by the System Development Life Cycle (SDLC).
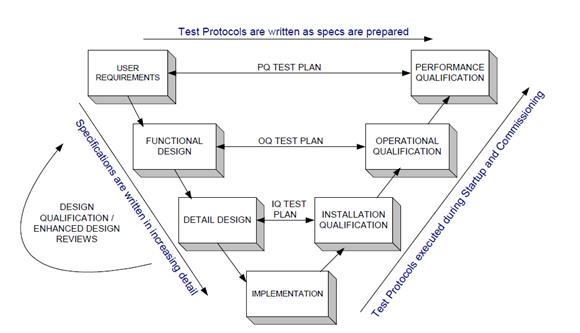
System Development Life Cycle can be divided into the following eight stages:
1、 Feasibility research
2、 Project plan
3、 Requirement definition
4、 System design
5、 System test
6、 System acceptance and confirmation
7、 use and maintenance
8、 System retire
Infrastructure
GAMP 5 Computerized System Software Classification
class 1:Infrastructure Software
class 3:Non-Configured Products
class 4:Configured Products
class 5:Custom Application
Computerized System Hardware Classification
class 1:Standard Hardware Components(At present, most of all kind of hardware used)
class 2:Custom Build Hardware Components(Such hardware is designed to meet the special needs and is the supplement to the standard hardware components)
Validation
System acceptance and validation, when the final computer systems and related files sent to the user, which is installed in the user's environment and to evaluate the correctness of its functions.
Qualification ("Installation Qualification ", "Operation Qualification" and "Performance Qualification") is complete and systematic testing behavior of computer system before the actual use, which directly affect the use quality of computer systems. That is, the "Qualification" is the last link of computer system quality assurance. Although some part of the "Qualification" is in progress under the same condition of unit testing and assembly testing, but "Qualification" is still necessary.
"Qualification" performed by users.
Key Activities During Qualification
1、 Executing the approved protocols
2、 Observe the results through protocol execution
3、 Document the observations
4、 Write execution report
5、 Approve the report, or follow Change Control process to re-execute the protocol (modified or approved)
21 CFR Part 11

Yenchen is able to provide document compliance with 21 CFR Part 11.
CFR:Code of Federal Regulations
21 CFR Part 11 is the United States Code of Federal Regulations Chapter 21, paragraph 11. The main purpose of this regulation is to develop specific provision of electronic record and electronic signature.
21 CFR Part 11
Electronic Records;Electronic Signatures
Subpart A – General Provisions
11.1 Scope
11.2 Implementation
11.3 Definitions
Subpart B – Electronic Records
11.10 Controls for closed systems
11.30 Controls for open systems
11.50 Signature manifestations
11.70 Signature/record linking
Subpart C – Electronic Signatures
General requirements
Electronic signatures and controls
Controls for identification codes/passwords
Yenchen is able to provide the above services.
Reference:GAMP5 and 21 CFR Part 11
Introduction of Computer System Validation and 21 CFR Part 11 | Tablet & Sterilization Machines - Pharmaceutical Manufacturing Equipment | Yenchen
Located in Taiwan since 1967, YENCHEN MACHINERY CO., LTD. has been a pharmaceutical manufacturing equipment supplier in pharmaceutical industry. Their main manufacturing and processing equipment include, hot air sterilizers, extrusion machines, tablet coating and compression machines, pellet making machines and oral solid dosage manufacturing equipment, which are sold to over 70 countries meeting international standards.
Yenchen was established in 1967, our products and services are widely used in the pharmaceutical, food, biotechnology, chemical, and cosmetic fields, which include solid dosage line, pellet machine production line, syrup line, injection line, ointment line, extraction & concentration turnkey equipments.
Yenchen has been offering customers high-quality pharmaceutical manufacturing equipment since 1967, both with advanced technology and 59 years of experience, Yenchen ensures each customer's demands are met.


